

Nanocrystal synthesis and structure fundamentals
Making nanocrystals with predictable properties requires control over both how they form and what they are made of. The Cossairt Lab investigates nucleation and growth from the earliest intermediates, especially atomically precise “magic-sized” clusters, through fully developed nanocrystals and heterostructures. A central theme is conversion chemistry: precursors transform into clusters, clusters transform into nanocrystals, and composition and oxidation state evolve during growth. We are interested in how subtle changes in speciation steer size, shape, crystal phase, and defect formation. We are interested in compositionally complex, heterostructured, and doped materials; templated growth using designed ligands and macromolecular interfaces; chirality encoded by inorganic-organic interfaces; and high-throughput experimentation to rapidly map synthetic parameter space. This work is enabled by complementary structural and spectroscopic tools, including UV-vis/PL, multinuclear NMR, single-crystal and powder XRD, X-ray spectroscopy, and electron microscopy, to make connections between what we make, what we measure, and what we ultimately can predict.
Key papers:

Quantum dot classical and quantum light
Quantum dots are versatile light emitters: efficient, tunable, and compatible with scalable processing. This makes them attractive for technologies spanning displays and lighting to sensing and quantum information science. In the Cossairt Lab, we design and characterize semiconductor nanocrystals that generate bright, stable classical emission across the visible and into the IR. A major focus is surface and interfacial chemistry: using atomically precise ligand design, rigid ligand networks, and intentionally engineered oxide or inorganic interlayers to control charge confinement, suppress nonradiative pathways, and improve spectral purity and photostability. For quantum light, we work toward solution-processable single-photon emitters that can be deterministically positioned and coupled to nano-optical cavities for on-chip photonics. We are especially interested in accessing a broad range of emission wavelengths, spanning the visible through the telecom, and growing these emitters to large sizes (> 50 nm) for ease of single-particle integration using methods like electrohydrodynamic inkjet printing. In collaboration with partners in IMOD and MEM-C, we connect synthesis, surface structure, and photophysics to device-relevant integration strategies to bring chemically programmable emitters into architectures that enable cavity-enhanced emission, improved collection efficiency, and scalable photonic platforms.
Key Papers:
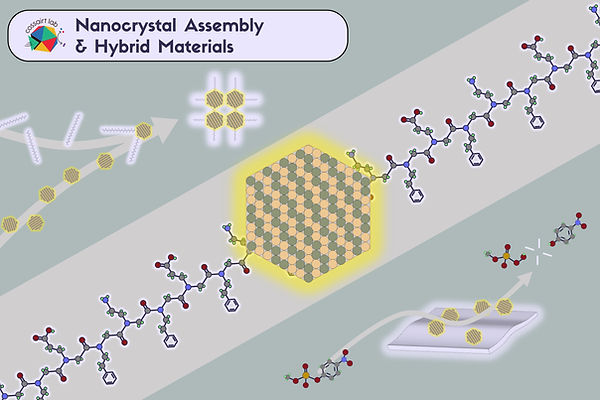
Nanocrystal assembly and hybrid materials
Natural materials achieve extraordinary function by organizing building blocks across length scales. Inspired by this, the Cossairt Lab develops bioinorganic hybrid assemblies that combine nanocrystals (quantum dots, metal oxides, and related building blocks) with sequence-defined macromolecules such as peptoids and designed proteins. By programming interactions at the molecular level, we aim to build 2D and 3D architectures with controlled spacing, orientation, and symmetry, key ingredients for collective optical and electronic behavior. Induced chirality encoded at the biomolecule/nanocrystal interface is a theme of particular interest. Another emerging direction is the creation of solution-processable optical metamaterials: assemblies whose emergent response (refractive index, chiroptical activity, light-matter coupling, and tailored scattering/absorption) arises from the designed arrangement of nanoscale components rather than their bulk composition alone. In all these efforts, advanced electron microscopy plays a central role in understanding building block structure and how assembly rules translate into function.
Key Papers: